
Gaseous State
Module I: Gaseous State (8 hrs)
[Prerequisites: Fundamentals of gaseous state. Postulates of kinetic theory of gases -
Derivation of kinetic gas equation - Maxwell's distribution of molecular velocities - Root
mean square, average and most probable velocities.]
Collision number - Mean free path - Collision diameter - Deviation from ideal behavior -
Compressibility factor – van der Waals equation of state (derivation required) - Virial
equation - Expression of van der Waals equation in virial form and calculation of Boyle
temperature - PV isotherms of real gases - Continuity of states - Isotherm of van der Waals
equation - Critical phenomena - Critical constants and their determination - Relationship
between critical constants and van der Waals constants
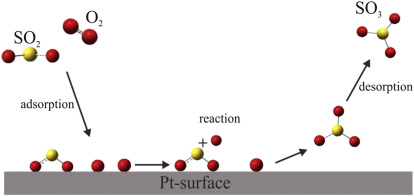
ADSORPTION AND CATALYSIS
Adsorption isotherms: Freundlich and Langmuir isotherms (derivation required) –
Multilayer adsorption – BET equation (derivation not needed) and its applications to
surface area measurements. Applications of adsorption.
Catalysis: Homogeneous and heterogenous catalysis – Theories of homogenous and
heterogenous catalysis – Enzyme catalysis – Michaelis-Menten equation (derivation not
required).

NON-AQUEOUS SOLVENTS
Non-aqueous Solvents: Classification – General properties – Self ionization and leveling
effect – Reactions in liquid ammonia, liquid N2O4, liquid SO2 and liquid HF.
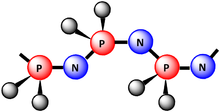
INORGANIC POLYMERS
Inorganic Polymers: Heterocatenation. Structure and applications of silicones and silicates.
Phosphazenes: Preparation, properties and structure of di and tri phosphonitrilic chlorides.
SN compounds: Preparation, properties and structure of S2N2, S4N4 and (SN)x.
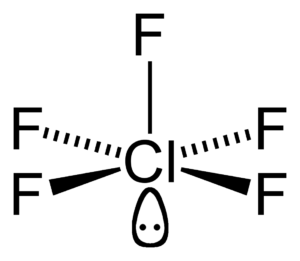
INTERHALOGEN COMPOUNDS
Electropositive character of iodine – General preparation and properties of interhalogen
compounds (study of individual members not required) – Structure, hybridization and
reactivity of ClF3, ICl3, IF5 and IF7 - Comparison of properties of halogens and
pseudohalogens (cyanogens as example) – Structure of polyhalide ions.
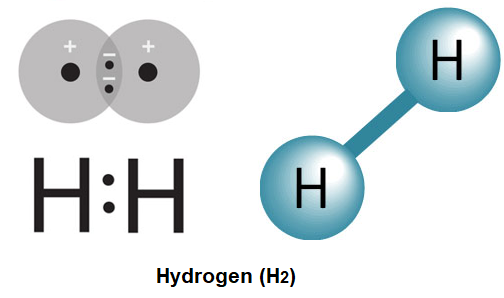
BONDING IN DIATOMIC MOLECULES
Quantum mechanical concept of bonding – (mixing of wave functions of different atoms).
Valence bond theory of H2 molecule (derivation not required). Molecular orbital theory of
H2
+
ion H2 molecule - linear combination of atomic orbitals (LCAO) and coefficients in the
linear combination (derivation not required). Potential energy diagram of H2 molecule
formation – equilibrium geometry. Bonding and antibonding molecular orbitals, bond order.
MO diagrams of homonuclear and heteronuclear diatomic molecules – He2, Li2, Be2, B2, C2,
N2, O2, F2, CO and NO. Comparison of VB and MO theories

molecular symmetry and group theory
: Module V: Molecular Symmetry and Group Theory (8 hrs)
Elements of symmetry of molecules (Identity, proper axis of rotation, plane of symmetry,
centre of symmetry and improper axis of rotation) – corresponding symmetry operations
– Schoenflies notation – binar y combinations of symmetry operations.
Rules for a set of elements to form a mathematical group - point group classification of
simple molecules – Cnv, Cnh, Dnh. Group multiplication table for C2v and C2h
SEMESTER III

Metallurgy
[Prerequisites: Occurrence of metals based on standard electrode potential – Concentration
of ores – Calcination and roasting – Reduction to free metal.]
Electrometallurgy – Hydrometallurgy. Refining of metals: Electrolytic refining, ion
exchange method, zone refining, vapour phase refining and oxidative refining – Ellingham
diagrams for metal oxides – Extractive metallurgy of Al, Fe, Ni, Cu, Ti and U. Alloys:
Definition – Composition and uses of German silver, brass, bronze, gunmetal and alnico.
Steel: Open hearth process – classification of steel – Composition and uses of alloy steels –
Composition, properties and applications of industrially important stainless steel types:
Austenitic, Martensitic and Ferritic stainless steels, Aerospace and automotive applications
of stainless steel. Intramedullary rods (a brief study)

PHASE EQUILIBRIA
Module III: Phase Equilibria (10 hrs)
[Prerequisites: Concept of phase - solid, liquid and gas - homogeneous and heterogeneous phase - component and degree of freedom.] Gibbs phase rule and its derivation. Clausius-Clapeyron equation and its applications to solidliquid, liquid-vapour and solid-vapour equilibria, phase diagram for one component systems, with applications. One component systems: Water and sulphur systems. Two component systems: Simple eutectic system (lead - silver system) – Pattinson’s process – Two component systems involving formation of compounds with congruent melting points (zinc-magnesium system and ferric chloride-water system) – Two component systems involving formation of compounds with incongruent melting points (sodium sulphate-water system). Freezing mixtures – Thermal analysis – Cooling curve method – Deliquescence and efflorescence. 44 Page 49 of 172 Liquid-liquid equilibria – Partially miscible and immiscible liquid systems – CST – Upper CST and lower CST – Steam distillation. Nernst distribution law: Derivation and applications.
References
th
1. B. R. Puri, L. R. Sharma, M. S. Pathania, Principles of Physical Chemistry, 46
Edn., Vishal Publishing Company, New Delhi, 2013.
2. P. W. Atkins, J. de Paula, Atkin’s Physical Chemistry, 8
th Edn., Oxford University Press,
2006.
3. Donald A. McQuarrie, John D. Simon, Physical Chemistry: A Molecular Approach,
University Science Books: Sausalito, CA; 1997.
rd
4. P. L. Soni, O. P. Dharmarha, U. N. Dash, Textbook of Physical Chemistry, 23
Sultan Chand & Sons, New Delhi, 2011.
Edn.,
Further reading
th
1. Gordon M. Barrow, Physical Chemistry, 5
Delhi, 2006.
Edn., Tata McGraw H

introductory Quantum Chemistry
Module II: Introductory Quantum Chemistry and the quantum mechanical model of the atom (10 hrs)
Operator algebra – linear and Hermitian operators, Laplacian and Hamiltonian operators,
eigen functions and eigen values of an operator. Non-commuting operators and the
Heisenberg's uncertainty principle.
Postulates of quantum mechanics. Well behaved functions. Time independent Schrödinger
wave equation for conservative systems. Application to particle in a one dimensional box –
normalization of wave function. Particle in a three dimensional box – separation of variables,
degeneracy.
Application of Schrödinger wave equation to hydrogen atom. The wave equation in spherical
polar coordinates. Separation of variables. Wave functions or atomic orbitals, radial and
angular parts of atomic orbitals. Quantum numbers (n, l, m). Radial functions, Radial
distribution functions and their plots, Angular functions and their plots (1s, 2s and2pz only).
The Stern-Gerlach experiment and the concept of electron spin, spin quantum number, spin
orbitals (elementary idea only). Pauli’s exclusion principle.

introductory Quantum Chemistry
Module II: Introductory Quantum Chemistry and the quantum mechanical model of the atom (10 hrs)
Operator algebra – linear and Hermitian operators, Laplacian and Hamiltonian operators,
eigen functions and eigen values of an operator. Non-commuting operators and the
Heisenberg's uncertainty principle.
Postulates of quantum mechanics. Well behaved functions. Time independent Schrödinger
wave equation for conservative systems. Application to particle in a one dimensional box –
normalization of wave function. Particle in a three dimensional box – separation of variables,
degeneracy.
Application of Schrödinger wave equation to hydrogen atom. The wave equation in spherical
polar coordinates. Separation of variables. Wave functions or atomic orbitals, radial and
angular parts of atomic orbitals. Quantum numbers (n, l, m). Radial functions, Radial
distribution functions and their plots, Angular functions and their plots (1s, 2s and2pz only).
The Stern-Gerlach experiment and the concept of electron spin, spin quantum number, spin
orbitals (elementary idea only). Pauli’s exclusion principle.
Semester 2 : Complementary Physics II
Semester 2 | Complementary Course II
PHY2C02: Optics, Laser & Electronics
36 hours (Credit - 2)

Alcohols and Phenols
Module I: Alcohols and Phenols (14 hrs)
[Prerequisites: Monohydric alcohols – Nomenclature, hydrogen bonding.]
Methods of formation of alcohols by reduction of carbonyl compounds.
Reaction of carbonyl compounds with Grignard reagent.
From alkenes (hydration, hydroboration oxidation and
oxymercuration-demercuration reactions). Reactions of alcohols: Acidic and basic nature of
alcohols, formation of ester, reaction with hydrogen halides (Lucas test), oxidation (with PCC
and KMnO4) – pinacol-pinacolone rearrangement (mechanism expected). Victor Meyer’s
test.
Phenols - Nomenclature, preparation of phenols (from cumene and aromatic sulphonic acid)
and acidity of phenol (substituent effects). Reactions of phenols – electrophilic aromatic
substitution (bromination, nitration and sulphonation) and carboxylation (Kolbe Schmitt
reaction). Riemer-Tiemann reaction (mechanism expected), Liebermann’s nitroso reaction
and Hauben-Hoesch reaction. Preparation of phenolphthalein and fluorescein and colour
change of phenolphthalein with pH.
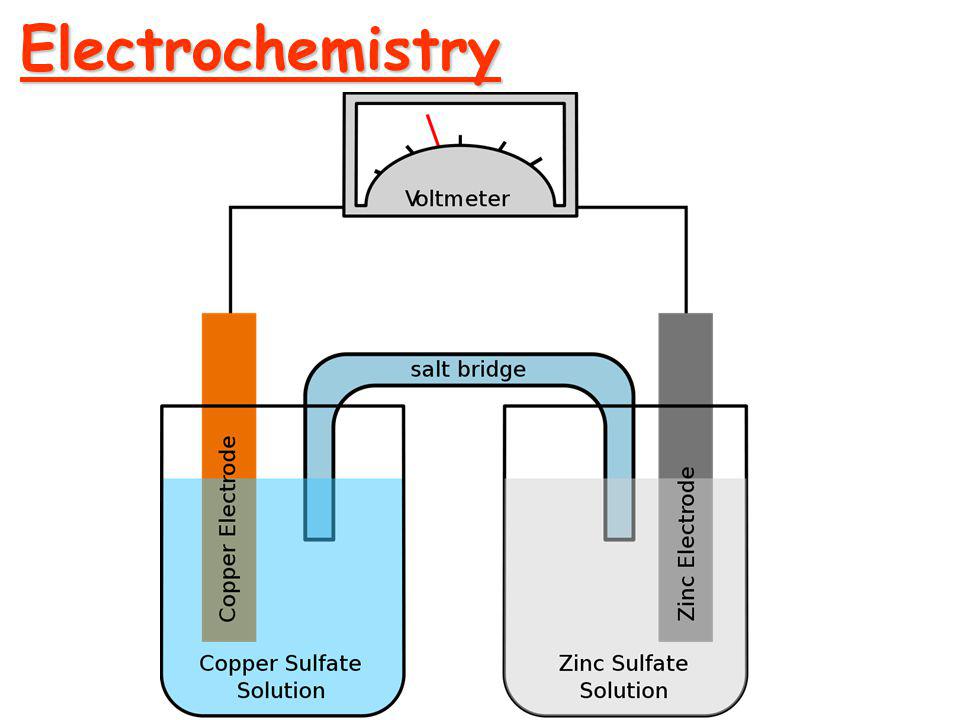
ELECTROCHEMISTRY
Specific conductance, equivalent conductance and molar conductance - Variation of
conductance with dilution - Kohlrausch's law - Degree of ionization of weak electrolytes -
Application of conductance measurements – Conductometric titrations.
Galvanic cells - Cell and electrode potentials - IUPAC sign convention – Reference
electrodes – Standard Hydrogen electrode – Calomel electrode - Standard electrode
potential - Nernst equation - H2-O2 fuel cell.
Ostwald's dilution law – Buffer solutions – Buffer action [acetic acid/sodium acetate &
NH4OH/NH4Cl], applications of buffers.

SOLID WASTE MANAGEMENT
House hold, municipal and industrial solid waste – Non-degradable, degradable and
biodegradable waste – Hazardous waste – Pollution due to plastics. Solid waste
management: Recycling, digestion, dumping, incineration, land treatment and composting.
Impacts of medical waste and e-waste and their disposal. Energy production from waste.